Cr (VI) Oxide Reagents
Mechanism + Description
Most chromate (VI) reagents work via a common mechanism – the formation of a chromate ester followed by the oxidative elimination of Cr (IV) and the oxidized alcohol product. The reaction is then repeated with the hydrate of the aldehyde.
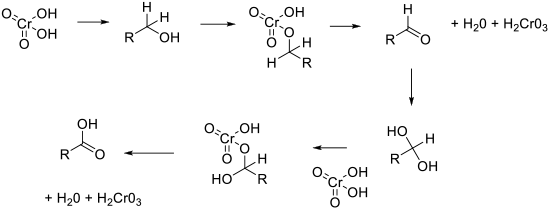
General comments
A number of Cr(VI) reagents will oxidase CH2OH to CO2H. The most frequently used is the Jones reagent – a dilute solution of CrO3 /H2SO4 – most commonly used in acetone solutions. The Jones reagent is generally more reactive than the Cornforth and Corey variants and may give issues with selectivity when using substrates with other oxidizable functional groups. The Corey and Schmidt variant uses pyridinium dichromate ((C5H5NH)2Cr2O7 ) in DMF. Absorbents like celite can be added to bind insoluble, lower valent Cr oxide by-products. Cr can be used catalytically with an added secondary oxidant to regenerate Cr(VI).
Key references
Catalytic CrO3 Oxidation with H5IO6: Zhao, M.; Li, J.; Song, Z.; Desmond, R.; Tschaen, D. M.; Grabowski, E. J. J.; Reider, P.J. A Novel Chromium Trioxide Catalyzed Oxidation of Primary Alcohols to the Carboxylic Acids. Tetrahedron Lett. 1998, 39 (30), 5323-5326.
Muzart, J. Chromium-Catalyzed Oxidations in Organic Synthesis. Chem. Rev. 1992, 92 (1), 113-40.
Oxidation with pyridinium dichromate in DMF: Corey, E. J.; Schmidt, G. Useful Procedures for the Oxidation of Alcohols Involving PyridiniumDichromate in Aprotic Media. Tetrahedron Lett. 1979, 20 (5), 399–402.
Relevant Scale-Up Examples with Scheme
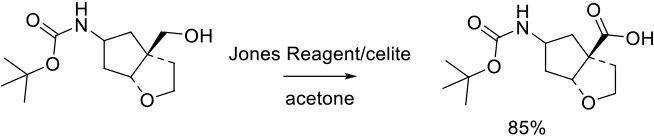
Org. Process Res. Dev. 2014, 18 (12), 1630−1640.
Experimental
265 g scale
Green Review
-
Atom efficiency (by-products Mwt)
Atom efficiency is generally poor if stoichiometric Cr reagents are used. The atom efficiency of catalytic reactions depends on the stoichiometric oxidant employed. - Safety Concerns
Chromium (VI) reagents strongly support combustion and can present an explosion hazard in contact with organic materials. Reactions can be very exothermic. - Toxicity and environmental/aquatic impact
Chromium (VI) compounds are both acutely toxic (irritant) and known to be carcinogenic to mammals; they are also very hazardous to aquatic organisms. Many Cr (VI) compounds are on the European Union REACH Substances of Very High Concern (SVAC) list due to their carcinogenic properties.
Toxic via skin, ingestion and inhalation.
Metal contamination of the final product needs to be considered. - Cost, availability & sustainable feedstocks
Chromium reagents are cheap and readily available though increasingly stringent legislation may make their use increasingly difficult.
European Chemicals Agency Candidate List of Substances of Very High Concern for Authorization. https://echa.europa.eu/candidate-list-table (accessed Sept. 2017).
https://www.epa.gov/sites/production/files/2016-09/documents/chromium-compounds.pdf - Sustainable implications
These reagents often form intractable residues after a reaction that can result in protracted cleaning procedures. All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Chromium is currently listed as being at low risk of depletion.