Metals and Air
Mechanism + Description
There may be a variety of mechanisms operating depending on the metal, phase (homo/hetero) and substrate. Most operate via the coordination of the alcohol to the metal, and the dehydrogenation to generate the aldehyde and a metal hydride. This is oxidized by O2 back to the active higher oxidation state species. Some systems may generate H2 and need hydrogen acceptors. The aldehyde is then oxidized to the acid; this could occur via a number of mechanisms, including radicals.
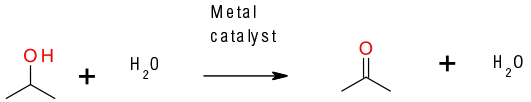
General comments
Though potentially one of the most atom efficient methods for the oxidation of alcohols to aldehydes, ketones and carboxylic acids, its success is tempered by the hazards of using air/O2 in the presence of flammable solvents. There is currently a focus on the use of flow/continuous processing to minimize/negate this hazard. A number of metals have been reported as showing activity in this transformation: V, Au, Pd, and Pt. Metal catalysts can be homo and heterogeneous. A recent advancement in this area is the use of water as an oxidant in the homogeneous catalyzed oxidation of primary alcohols to carboxylic acids.
Key references
Technology Solutions for Oxidation with O2/Air
Relevant Scale-Up Examples with Scheme
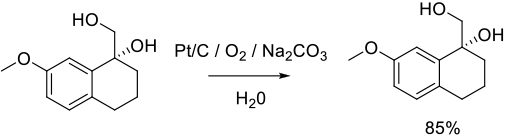
Org. Process Res. Dev. 2003, 7 (2), 198-201.
Experimental
16 g scale
Green Review
-
Atom efficiency (by-products Mwt)
The exact atom efficiency cannot be determined given the range of metal-air conditions, however, in general, molecular oxygen will be one of the greenest conditions available if coupled with a catalytic metal. - Safety Concerns
The use of high temperatures and flammable solvents in oxygen-containing atmospheres should be given due consideration. - Toxicity and environmental/aquatic impact
Varying metals have different human toxicity and ecotoxicity issues. In all cases, the use of catalytic quantities should be beneficial, and recovery (and zero release) of precious metals is advised for both environmental and financial reasons. - Cost, availability & sustainable feedstocks
Many of the metals outlined are simple complexes with good commercial availability on scale, however, some of them are more novel (nano-clusters, complexes, etc.) and in their infancy. - Sustainable implications
This can potentially be a very green oxidation technology if the hazards are managed. If possible, base metals should be used as catalysts. Precious metals have a high LCI to produce and several, like Ir and Pd, are on the medium- to high-risk level for depletion.