Activated Hydrogen Peroxide/Hydroperoxides
Mechanism + Description
The mechanism may vary with the metal and homo/hetero nature of the catalyst. With homogeneous catalysts, several reports have described that O2 generated via the decomposition of H2O2 is not the oxidant. For homogeneous reactions, the active catalyst is probably a peroxy metal species.
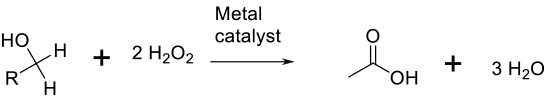
General comments
The method of activating hydrogen peroxide/hydroperoxides usually on metal catalysts is a variant on the use of air/O2. The reaction can be carried out under mild conditions, but the background decomposition of peroxide or hydroperoxide can lead to higher-than-stoichiometric demands for the oxidant. H2O2 is an attractive oxidant since the by-product is water. Other functional groups may be oxidized.
Key references
Relevant Scale-Up Examples with Scheme
No scale-up examples identified.