Catalytic Hypervalent Iodine Catalysts
Mechanism + Description
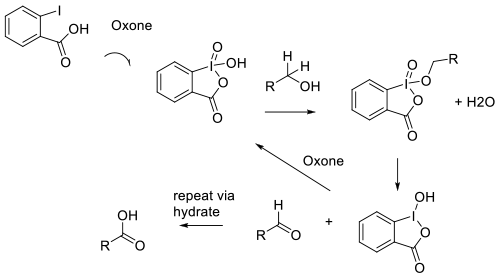 The activation of the aryl iodide catalyst by
oxidation ‘in situ‘ by Oxone, followed by
the expected oxidation mechanism for IBX/
Dess-Martin reagents. The reduced catalyst
is re-oxidized to the high valent active form
and the aldehyde product is further oxidized via
the hydrate to the acid.
The activation of the aryl iodide catalyst by
oxidation ‘in situ‘ by Oxone, followed by
the expected oxidation mechanism for IBX/
Dess-Martin reagents. The reduced catalyst
is re-oxidized to the high valent active form
and the aldehyde product is further oxidized via
the hydrate to the acid.
General comments
A number of examples have been published using terminal oxidants (mainly Oxone) to generate hypervalent iodine compounds in situ. These react very much as stoichiometric IBX/Dess Martin reagents. The oxidation of the primary alcohol to the aldehyde occurs, followed by a second oxidation of the aldehyde hydrate. These catalytic methods avoid having to use isolated unstable/explosive hypervalent iodine reagents.
Key references
Relevant Scale-Up Examples with Scheme
No scale-up examples identified.