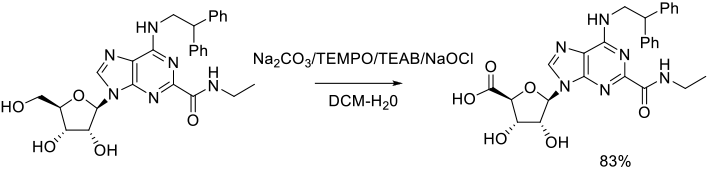Mechanism + Description
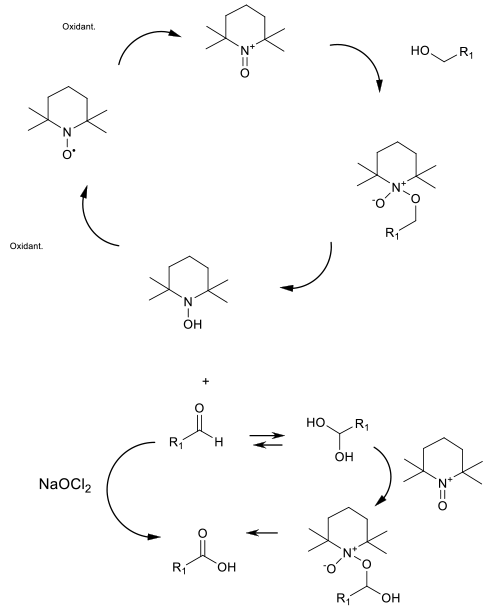 The oxonium ion generated by the oxidation of the
TEMPO radical is the oxidant. Used catalytically,
a stoichiometric oxidant like bleach or O2 (air) is
used to drive the catalytic cycle. The oxidation to
aldehyde is rapid. The oxonium ion will only oxidize
the aldehyde hydrate, so for substrates with a negligible
degree of hydration, a second oxidant – usually NaOCl2
– is added.
The oxonium ion generated by the oxidation of the
TEMPO radical is the oxidant. Used catalytically,
a stoichiometric oxidant like bleach or O2 (air) is
used to drive the catalytic cycle. The oxidation to
aldehyde is rapid. The oxonium ion will only oxidize
the aldehyde hydrate, so for substrates with a negligible
degree of hydration, a second oxidant – usually NaOCl2
– is added.
General comments
A common terminal oxidant is bleach (NaOCl), which is often employed with a bromide or borate co-catalyst. Reactions in water of bi-phasic reactions are often helped by the addition of a phase transfer catalyst. Ring opening of the oxonium ion and deoxygenation of the TEMPO catalyst can limit catalytic efficiency. In some cases, other catalytic oxidants like copper salts or laccase enzymes are added to promote the TEMPO oxidation, especially when air is used as the stoichiometric oxidant. Because of its hydrophobic nature and steric hindrance, TEMPO oxidations can be kinetically slow. A number of related, but much more reactive nitroxyl radical catalysts like ABNO,AZADO and 1-methylAZADO are becoming more commercially available. While these are currently much more expensive than TEMPO on a mole basis, they are often several of orders more reactive and can be dosed at much lower levels than TEMPO. A number of solid-supported TEMPO or TEMPO analogues are available commercially. These can facilitate downstream processing, provide for reusable catalysts, and facilitate chemistry in flow reactors. Considerations should be given to their preparation and lifespan (number of reuses before depletion of results) to make an informed decision on whether it is a better option than the standalone/homogeneous reagents.
Key references
Moriyama, K.; Takemura, M.; Togo, H. Selective Oxidation of Alcohols with Alkali Metal Bromides as Bromide Catalysts: Experimental Study of the ReactionMechanism. J. Org. Chem. 2014, 79 (13), 6094-6104.
Ciriminna, R.; Pagliaro, M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Org. Process Res. Dev.2010, 14 (1), 245–251.
Zhao, M.; Li, J.; Mano, E.; Song, Z.; Tschaen, D. M.; Grabowski, E. J. J.; Reider, P. J. Oxidation of Primary Alcohols to Carboxylic Acids with Sodium Chlorite Catalyzed by TEMPO and Bleach. J. Org. Chem. 1999, 64 (7), 2564–2566.
Yakura, T.; Ozono, A. Novel 2,2,6,6-Tetramethylpiperidine 1-Oxyl–Iodobenzene Hybrid Catalyst for Oxidation of Primary Alcohols to Carboxylic Acids. Adv. Synth. Catal. 2011, 353 (6), 855–859.
Galletti, P.; Pori, M.; Funiciello, F.; Soldati, R.; Ballardini, A.; Giacomini, D. Laccase-Mediator System for Alcohol Oxidation to Carbonyls or Carboxylic Acids: Toward a Sustainable Synthesis of Profens. ChemSusChem.2014, 7 (9), 2684-2689.
Shibuya, M.; Sato, T.; Tomizawa, M.; Iwabuchi, Y. OxoammoniumSalt/NaClO2: An Expedient, Catalytic System for One-Pot Oxidation of Primary Alcohols to Carboxylic Acids with Broad Substrate Applicability. Chem. Commun. 2009, 0, 1739-1741.
Galletti, P.; Pori, M.; Giacomini, D. One-Step Oxidation of 2-Arylpropanols to 2-Arylpropionic Acids: Improving Sustainability in the Synthesis of Profens. Synlett. 2010, 17, 2644-2648.
Shibuya, M.; Doi, R.; Shibuta, T.; Uesugi, S.; Iwabuchi, Y. Organocatalytic One-Pot Oxidative Cleavage of Terminal Diols to Dehomologated Carboxylic Acids. Org. Lett. 2012, 14 (19), 5006-5009.
Zhao, M. M.; Li, J.; Mano, E.; Song, Z. J.; Tschaen, D. M. Oxidation of Primary Alcohols to Carboxylic Acids with Sodium Chlorite Catalyzed by Tempo and Bleach: 4-Methoxyphenylacetic Acid. Org. Synth. 2005, 81, 195.
TEMPO Analogues
Shibuya, M.; Tomizawa, M.; Suzuki, I.; Iwabuchi, Y. 2-Azaadamantane N-Oxyl (AZADO) and 1-Me-AZADO: Highly Efficient Organocatalysts for Oxidation of Alcohols. J. Am. Chem. Soc. 2006, 128(26), 8412–8413.
Shibuya, M; Osada, Y.; Sasano, Y.; Tomizawa, M.; Iwabuchi, Y. Highly Efficient, Organocatalytic Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2011, 133 (17), 6497–6500.
Kuang, Y.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. Solvent Free Aerobic Oxidation of Alcohols with 1-Methyl-2-Azaadamantane N-Oxylas a Recyclable Catalyst Through Phase Separation. Green Chem. 2011, 13, 1659-1663.
Shibuya, M.; Tomizawa, M.; Sasano, Y.; Iwabuchi, Y. An Expeditious Entry to 9-Azabicyclo[3.3.1]nonane N-Oxyl (ABNO): Another Highly Active Organocatalyst for Oxidation of Alcohols. J. Org. Chem. 2009, 74 (12), 4619–4622.
Hayashi, M.; Sasano, Y.; Nagasawa, S.; Shibuya, M.; Iwabuchi, Y. 9-Azanoradamantane N-Oxyl (Nor-AZADO): A Highly Active Organocatalyst for Alcohol Oxidation. Chem. Pharm. Bull. 2011, 59 (12), 1570-1573.
Solid Supported TEMPO Catalysts
Dijksman, A.; Arends, I. W. C. E.; Sheldon, R. A. A Comparison of the Activity of Polymer Immobilised TEMPO (PIPO) with MCM-41 and Silica Supported TEMPO as Heterogeneous Catalysts for the Oxidation of Alcohols. Synlett. 2001, 1, 102-104.
Dijksman, A.; Arends, I. W. C. E.; Sheldon, R. A. Polymer Immobilized TEMPO (PIPO): An Efficient Catalyst for the Chlorinated Hydrocarbon Solvent-Free and Bromide-Free Oxidation of Alcohols with Hypochlorite. Chem. Commun. 2000, 0, 271-272.
Karimi, B.; Farhangi, E. A Highly Recyclable Magnetic Core-Shell Nanoparticle-Supported TEMPO Catalyst for Efficient Metal- and Halogen-Free Aerobic Oxidation of Alcohols in Water. Chem.–Eur. J. 2011, 17 (22), 6056-6060.
Roy, M.; Poupon, J.; Charette, A. B. Tetraarylphosphonium Salts as Soluble Supports for Oxidative Catalysts and Reagents. J. Org. Chem. 2009, 74 (22), 8510–8515.
Karimi, B.; Biglari, A.; Clark, J. H.; Budarin, V. Green, Transition-Metal-Free Aerobic Oxidation of Alcohols Using a Highly Durable Supported Organocatalyst. AngewandteChemie International Edition. 2007, 46 (38), 7210-7213.
Matsumoto, K.; Iwata, T.; Suenaga, M.; Okudomi, M.; Nogawa, M.; Nakano, M.; Sugahara, A.; Bannai, Y.; Baba, K. Mild Oxidation of Alcohols Using Soluble Polymer-Supported TEMPO in Combination with Oxone: Effect of a Basic Matrix of TEMPO Derivatives. Heterocycles. 2010, 81 (11), 2539-2553.
Michaud, A.; Gingras, G.; Morin, M.; Béland, F.; Ciriminna, R.; Avnir, D.; Pagliaro, M. SiliaCat TEMPO: An Effective and Useful Oxidizing Catalyst. Org. Process Res. Dev. 2007, 11 (4), 766–768.
Steves, J. E.; Preger, Y.; Martinelli, J. R.; Welch, C. J.; Root, T. W.; Hawkins, J. M.; Stahl, S. S. Process Development of CuI/ABNO/NMI-Catalyzed Aerobic Alcohol Oxidation. Org. Process Res. Dev. 2015, 19 (11), 858-864.
Relevant Scale-Up Examples with Scheme
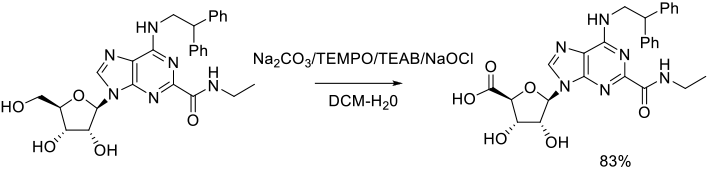
Org. Process Res. Dev. 2012, 16 (3), 470−483.
Experimental
36 kg scale
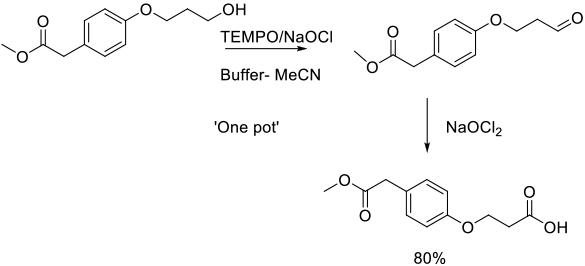
Org. Process Res. Dev. 2012, 16 (4), 625−634.
Experimental
1.4 kg scale
Green Review
-
Atom efficiency (by-products Mwt)
Generally good if optimized for charge of TEMPO or other catalyst. Mainly driven by stoichiometric/terminal oxidant. NaOCl gives NaCl as a by-product. Air will give H2O2 or H2O as a by-product. Atom efficacy can be poor with high Ml. Wt. oxidants like oxone or high valent iodine reagents.
- Safety Concerns
All TEMPO oxidations are exothermic and may present delayed exotherms. Compatibility of NaOCl with other reaction components needs to be considered.
- Toxicity and environmental/aquatic impact
Though toxicity and environmental/aquatic impacts are generally low when used catalytically, the major concerns arise from the co-oxidant. Nitroxyl radicals like TEMPO and the hydroxylamine intermediates in the oxidation cycle give positive structural alerts as potential genotoxic impurities (PGI).
Strohmeyer, H. E.; Sluggett, G. W. Determination and Control of TEMPO, a Potentially Genotoxic Free Radical Reagent Used in the Synthesis of Filibuvir. J. Pharm. Biomed. Anal. 2012, 62 (25), 216– 219.
- Cost, availability & sustainable feedstocks
The cost of TEMPO has fallen over time and is now available in bulk. Other analogues are less commercially available and much more expensive, but could show far greater activity. The skeleton of TEMPO comes from acetone and ammonia.
- Sustainable implications
With good optimization of catalyst loading and a low molecular weight terminal oxidant like NaOCl, this oxidation is a good choice. The major concern would be the solvent used. Many initial publications used dichloromethane, but later work has shown that more sustainable solvents can be used (see references).
 The oxonium ion generated by the oxidation of the
TEMPO radical is the oxidant. Used catalytically,
a stoichiometric oxidant like bleach or O2 (air) is
used to drive the catalytic cycle. The oxidation to
aldehyde is rapid. The oxonium ion will only oxidize
the aldehyde hydrate, so for substrates with a negligible
degree of hydration, a second oxidant – usually NaOCl2
– is added.
The oxonium ion generated by the oxidation of the
TEMPO radical is the oxidant. Used catalytically,
a stoichiometric oxidant like bleach or O2 (air) is
used to drive the catalytic cycle. The oxidation to
aldehyde is rapid. The oxonium ion will only oxidize
the aldehyde hydrate, so for substrates with a negligible
degree of hydration, a second oxidant – usually NaOCl2
– is added.