Hydrolases
Mechanism + Description
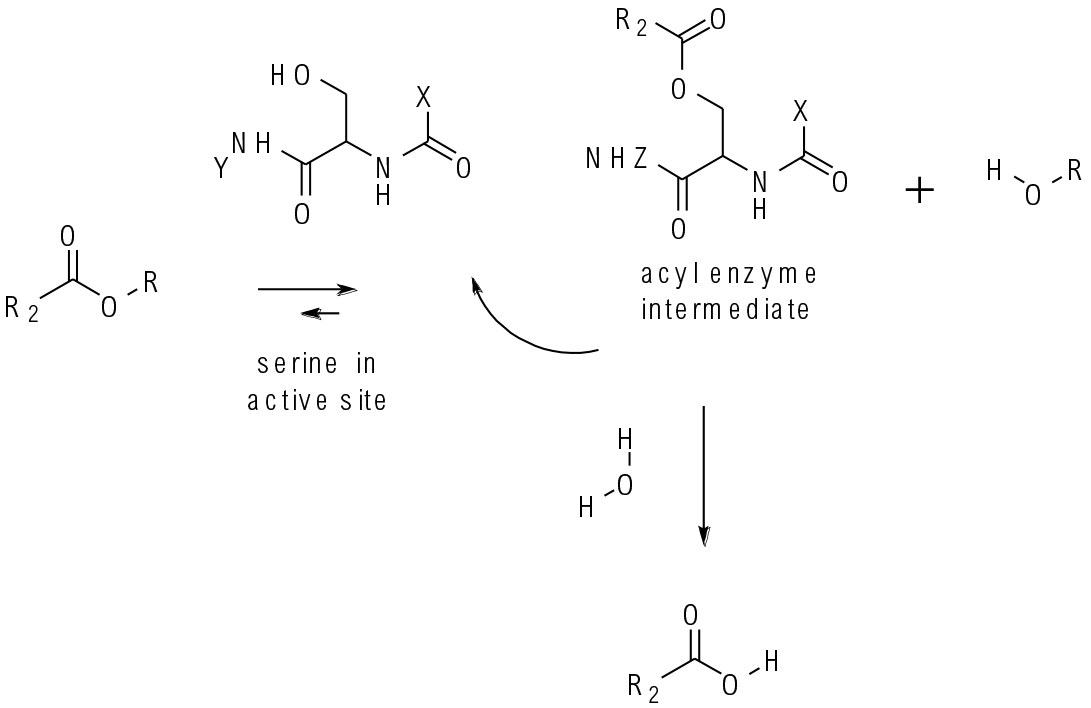
Most hydrolases work via formation of an acyl intermediate bound to serine or cysteine in the active site of the enzyme. Other amino acids in the active site are responsible for the observed rate acceleration. Chiral differentiation is determined by the 3D shape of the active site.
General comments
A wide class containing esterases, lipases and proteases-amidases. These enzymes manipulate ester and amide groups via hydrolysis, and certainly in some cases under specific conditions will work in reverse mode, for example, creating esters and amides. The hydrolase class was the first to be widely adopted by synthetic organic chemists and represents a fairly mature area now, with many industrial processes having been reported, primarily in the kinetic resolution of racemates.
Many hydrolases will work on substrates under conditions where the unwanted enantiomer will racemise (base, another enzyme, metal-based catalyst) thus giving a dynamic kinetic resolution. Many hydrolases will also give good results in the desymmetrisation of meso compounds giving single enantiomer products in high ee. Whilst most hydrolases are used in processes to produce chiral molecules, they can be usefully employed in the selective hydrolysis of esters amides in molecules with multiple hydrolysable groups that would also be hydrolysed by acid/base catalysis.
Key references
See also the biocatalysis section of Ester Deprotection Guide.
Müller, M. Enzymatic Synthesis of Tertiary Alcohols. ChemBioEng Rev. 2014, 1 (1), 14–26.
Långvik, O.; Saloranta, T.; Murzin, D. Y.; Leino, R. Heterogeneous Chemoenzymatic Catalyst Combinations for One-Pot Dynamic Kinetic Resolution Applications. ChemCatChem 2015, 7, 4004–4015.
Ahmed, M.; Kelly, T.; Ghanem, A. Applications of enzymatic and non-enzymatic methods to access enantiomerically pure compounds using kinetic resolution and racemisation. Tetrahedron 2012, 68, 6781–6802.
Relevant scale up examples

Org. Process Res. Dev. 2002, 6, 488-491
80 kg scale

Org. Process Res. Dev. 2003, 7, 663-675
250 kg scale
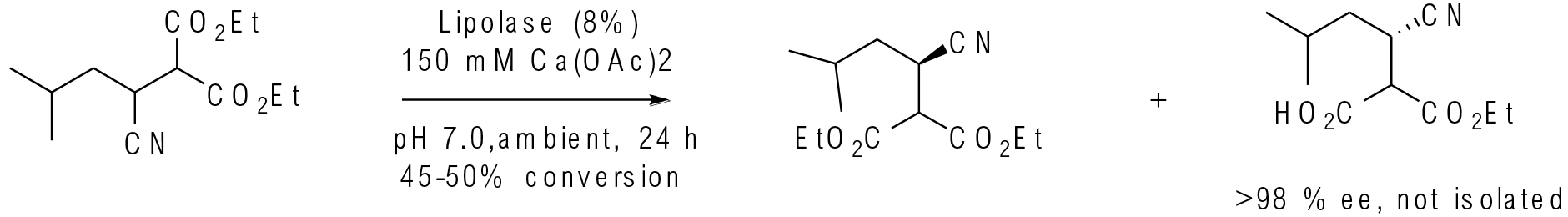
Org. Process Res. Dev. 2008, 12, 392-398
10,000 kg scale
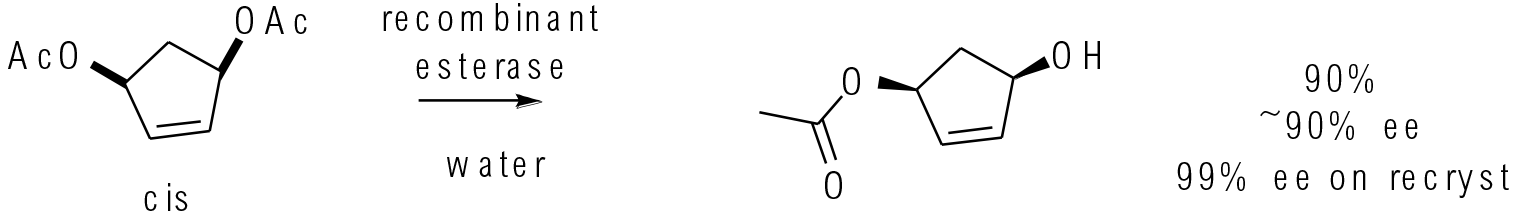
Org. Process Res. Dev. 2016, 20,1258-1264
80 g scale
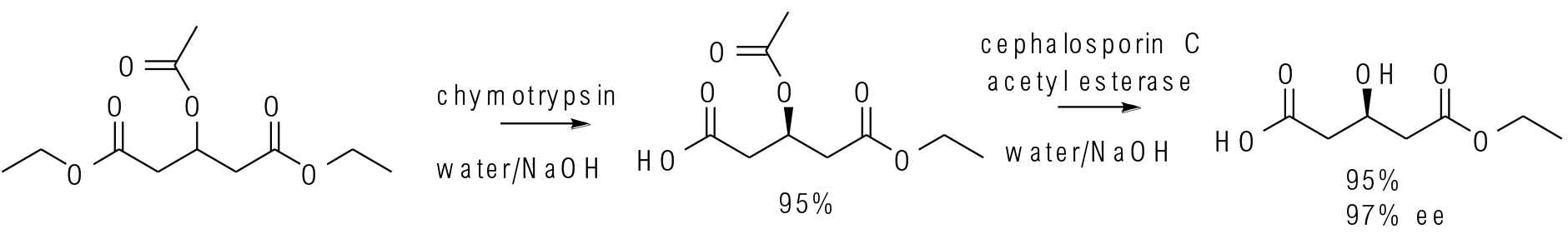
Org. Process Res. Dev. 2015, 19, 635−638
10 g scale

Org. Process Res. Dev. 2006, 10, 644−649
10 kg scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.